Weekly Medtech Pro Report: The Neuromodulation Devices Market, Radical Neck Dissection Procedures, and Startup Spotlight


Life Science Intelligence’s Weekly Medtech Pro Review provides a preview of select market data and startups covered in the full Medtech Pro Platform, which is a comprehensive market intelligence solution for medtech executives. As a preview of the types of content found on Medtech Pro, the Weekly Medtech Pro Review will cover select market data, procedure volumes, and startups.
Click here to learn more or subscribe to the full Medtech Pro platform.
Medtech Market Snapshot – Neuromodulation Devices
Neuromodulation devices, alter the electrical signals in the body with the intention of improving function or reducing pain in the body. There are multiple types of neuromodulation devices in use today, including implantable, transcutaneous, and transcranial devices. Neuromodulation devices are often used to treat patients who have failed to respond to pharmacological management of their condition.
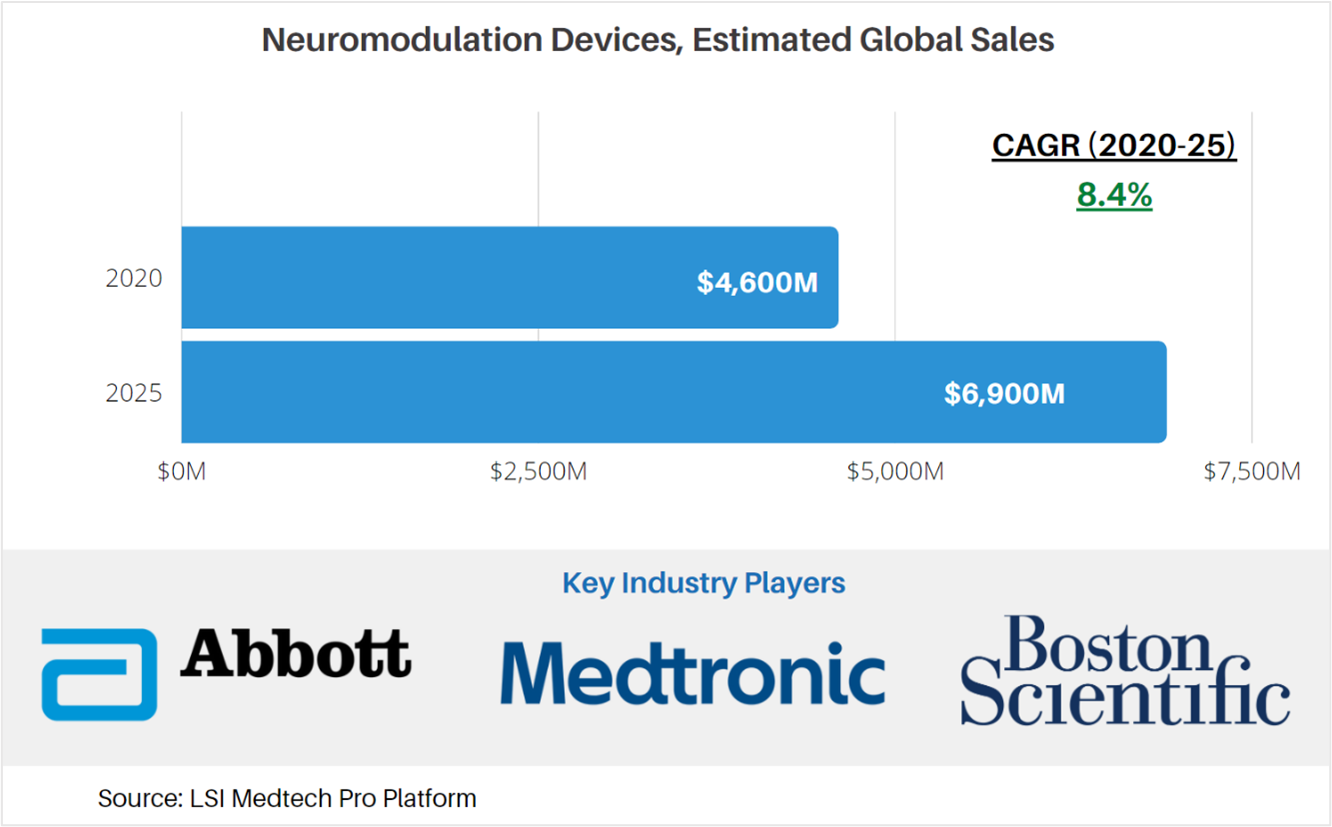
According to market data from LSI, global sales attributable to neuromodulation devices were approximately $4.6B in 2020. The market for these products is projected to increase at a CAGR of 8.4% to $6.9B in 2025. As is the case with many medtech markets, unit sales and implantation procedure volumes for neuromodulation devices were significantly impacted by the COVID-19 pandemic.
Products included in LSI’s assessment of the global neuromodulation devices market include transcutaneous and implantable devices. Implantable devices account for approximately 75% of total neuromodulation device sales.
For more market data on the global market for neuromodulation devices, as well as other medtech markets, visit LSI’s Medtech Pro platform.
Surgical Procedure Volumes – Radical Neck Dissection in the UK
Life Science Intelligence tracks surgical procedure volumes across 37 countries and 12 major surgical markets (e.g. orthopedics, ENT, aesthetics). This week, our featured surgical procedure volume is for radical neck dissections performed in the UK. Radical neck dissection is considered the “gold standard” for the treatment of head and neck cancers and metastatic lymph nodes. Neck dissection can be modified to be more conservation or radical, depending on clinical presentation of metastatic disease. Any neck dissection involves the excision of soft tissue in the neck and the lymph nodes contained therein. Modifications of radical neck dissection are done to preserve one or more non-lymphatic structures.
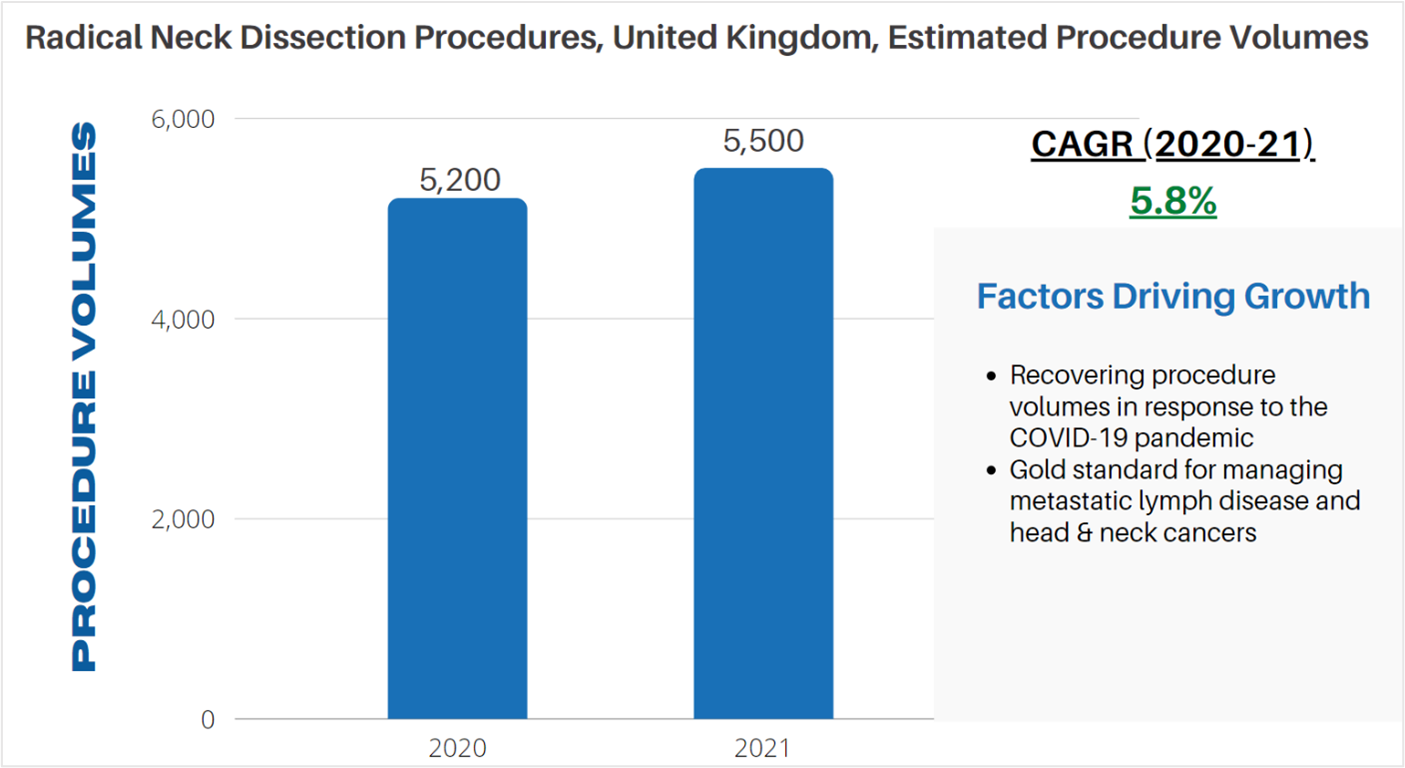
According to LSI’s Global Surgical Procedure Volumes database there were an estimated 5,200 radial neck dissections performed in 2020 in the UK. The COVID-19 pandemic had a restraining effect on radical neck dissections, largely due to a reduction in surgical capacity and a reluctance from patients to have surgery during the height of the pandemic. A recovery in procedure volumes is projected to occur in 2021, with LSI estimating that 5,500 procedures will be performed in the UK. Following this recovery from the pandemic, radical neck dissection procedures are projected to increase in line with epidemiologic trends for head and neck cancers.
Startup Spotlight

To see AiM Medical Robotics presentation at the LSI Emerging Medtech Summit, click here.
Despite the improvements in pre-operative imaging and mapping technologies, many challenges still necessitate the need for removals during surgery and repeat revisions. To improve neurosurgical outcomes, AiM Medical Robotics is developing a robotic system that is compatible with intraoperative magnetic resonance imaging (MRI).
Licensed from technology developed at Worcester Polytechnic Institute, AiM’s robotic system is intended to be portable system that will enable real-time guidance of therapy delivery. The system will use a needle-based ultrasonic device to safely ablate tumors and structures of the brain causing functional neurological disorders (e.g. epilepsy). Current neurosurgical solutions involve the use of harmful radiation-based technologies (e.g. wide-beam radiation therapy) or lasers, which can cause damage to surrounding tissue.
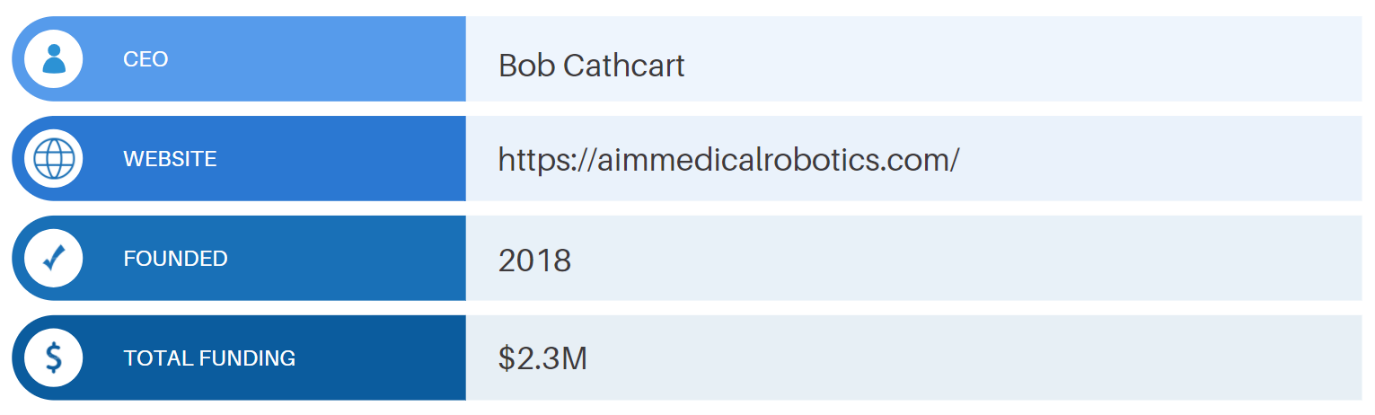
The company has raised approximately $2.3M in funding. AiM Medical Robotics is planning to bring the robot to market in 2023.

Life Science Intelligence is proud to announce that Proprio will be a presenting at the 2022 Emerging Medtech Summit in Dana Point, California.
Proprio is developing a digital surgery platform to augment the capabilities of surgeons. The company’s goal is to reduce the cognitive and ergonomic load on the surgeon, while providing them with 3D images that offer more dynamic information compared to standard 2D images captured from legacy surgical imaging technologies.
The PARADIGM system is a real-time multimodal imaging device that utilizes artificial intelligence to reconstruct 2D images into 3D. The digital reconstruction is then visible to the surgeon through a wearable headset. Tracking technology in the surgeon’s headset will allow the system to give the surgeon 3D data specific to wear the surgeon is looking. Proprio’s system will provide surgeons with pre-operative imaging, virtual annotation, collaborative feedback, and improved magnification through – all within the surgeon’s field-of-view. Post-surgery, the data from procedures performed using Proprio’s technology will be available for review and teaching via simulation.
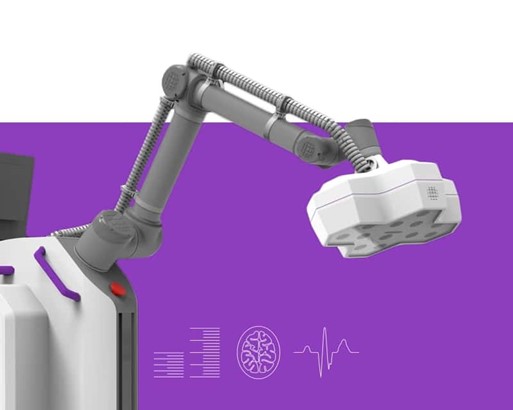
Figure 1: Proprio PARADIGM system
To date, Proprio has raised $42.1M in funding. Key investors include virtual reality hardware developer HTC and tech-giant Intel, among other investor groups.
Currently, Proprio is evaluating the use of their technology in neuro and orthopedic surgery. The company’s product is currently pending FDA clearance.

San Diego-based Biolinq is developing a pain-free solution for the measurement of blood glucose levels. While major strategics such as Abbott, Dexcom, and Medtronic offer needle-free continuous glucose monitors (CGMs), Biolinq’s device will further miniaturize the technology that is changing the way diabetics manage their disease.
The company’s technology, which was spun out from UC San Diego, is a coin-sized patch that utilizes proprietary microarray technology to measure glucose found in the interstitial fluid between cells underneath the skin. In addition to its smaller size, Biolinq’s biosensor further distinguishes itself by not requiring a needle or applicator to place the sensor beneath the skin – the company’s technology rests on top of the capillary bed in the skin.
Biolinq plans to expand the monitoring capabilities of their biosensor, purporting that their electrochemical sensor can monitor other biomarkers.
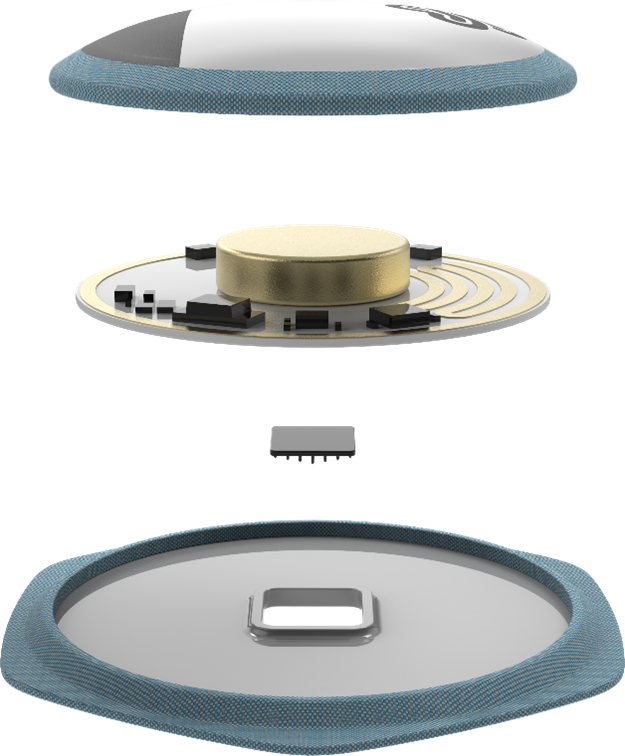
Figure 2: Biolinq glucose biosensor
In November 2021, Biolinq announced that it had secured a $100M investment from a Series B round. According to the company, the funding will help advance their technology through the regulatory review process and begin commercialization.








